Engine Sensor,Deutz Engine Sensor,Oil Pressure Sensor,Deutz Oil Sensor ACRO (TIANJIN) INTERNATIONAL TRADE CO., LTD , https://www.acrospareparts.com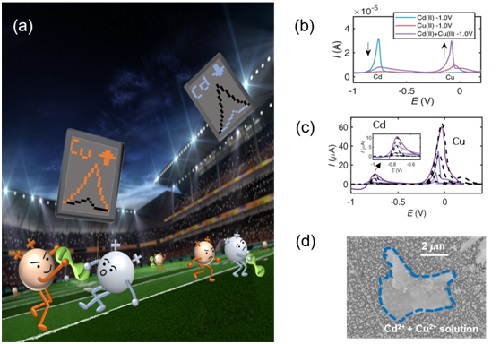
[ Instrument Network Instrument Development ] Recently, researchers from the Institute of Hefei Institute of Material Science, Chinese Academy of Sciences, Huang Xingjiu and Associate Professor Lin Chuhong made new discoveries in the joint detection of heavy metals in water: by combining electrochemical experiments with electrode process dynamics simulation calculations, explained Electrochemical analysis detects the mutual interference mechanism of heavy metals Cd(II) and Cu(II). The related results were published in the American Chemical Society Journal of Analytical Chemistry under the title of Metal Replacement Causing Interference in Stripping Analysis of Multiple Heavy Metal Analytes: Kinetic Study on Cd (II) and Cu (II) Electroanalysis via Experiment and Simulation.
(a) Schematic diagram of heavy metal Cd(II), Cu(II) co-deposition; (b) Anode voltammetric signal when Cd, Cu and both are jointly detected; (c) Electrochemical signal when Cd and Cu are jointly detected (real) Line) and theoretical simulation (dashed line); (d) scanning tunneling micrograph of co-deposited Cd, Cu, blue line marking Cd deposit
The electric analysis method can quickly and quantitatively detect trace heavy metal ions in water, and has been widely concerned by research and development at home and abroad. However, the detection of multiple coexisting heavy metal ions in water has long been an inevitable mutual interference problem. At present, the cause of the interference mechanism in the presence of multi-component heavy metals is still unclear. The mutual interference of multiple components often causes the concentration of analytes. Misjudgment and incorrect selection of electrode materials greatly hinder the effectiveness and reliability of electroanalytical methods for detecting heavy metal ions.
It is reported in the literature that when the dissolution potential is >200mV, the dissolution signals should not affect each other, but on the unmodified glassy carbon electrode, the researchers found that the heavy metal ions Cd(II) and Cu(II) with a difference of 700mV in the dissolution potential were detected together. Signal interference still occurs, and the weakening of the Cd signal and the substantial increase of the Cu signal are observed. In order to explain this phenomenon, the intelligent researchers obtained the kinetic parameter changes of Cd(II) and Cu(II) in the process of separate detection and common detection through the simulation simulation of the anode square wave dissolution signal, and found that Cd and Cu are common. The main reason for signal interference in the detection is that Cu(II) ions in the co-deposition process replace Cd which has been deposited on the surface of the electrode, resulting in a decrease in the amount of dissolved Cd and an increase in the amount of Cu. This basic research on heavy metal electroanalytical detection not only provides the mechanism of signal interference caused by heavy metal ions co-deposition, but also provides effective theoretical guidance for designing anti-interference detection interface.
The work has been approved by the National Natural Science Foundation of China, the Youth Project, the Chinese Academy of Sciences Hundred Talents Program, the Postdoctoral Innovative Talent Support Program, the Hefei Institute of Material Science, the 13th Five-Year Plan of the Chinese Academy of Sciences, and the Natural Science Foundation of Anhui Province. stand by.
(Original title: New progress in the research on the interference mechanism of intelligent analysis of multiple heavy metals in water)